Clinical Studies
Oral Mucositis Overview1
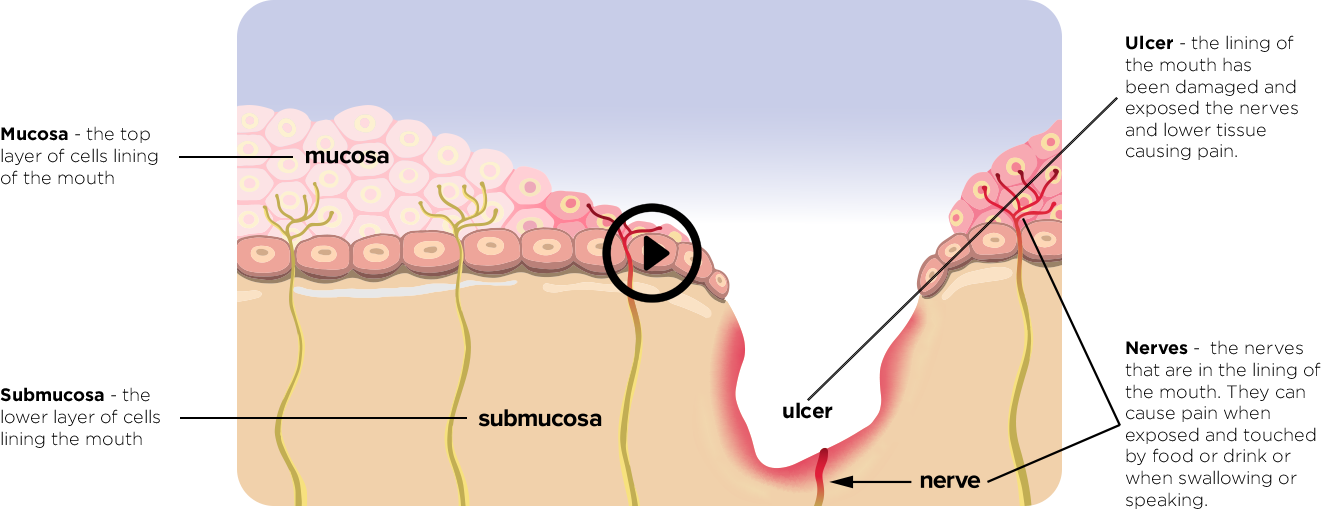
One of the side effects of cancer therapies is oral mucositis, a debilitating condition resulting from the destructive effects of chemotherapeutic drugs and radiation on the oral mucosa. The damaged lining of the oral cavity usually presents as erythema, swelling, and ulceration. The mucositis may be so severe that patients’ food and fluid intake and speech are reduced, further compromising the patients’ response to treatment. The injured oral mucosa and lowered immunity from chemotherapy and radiotherapy make the patient more vulnerable to opportunistic infections in the mouth and provide a possible portal of entry for microorganisms to get into systemic circulation. Systemic infection may lead to complications, interruption of therapy and hospitalization with parenteral nutritional therapy and opioid analgesics.
Oral Mucositis Occurs in2:
Chemotherapy patients
| 20% to 40% |
Patients being treated for solid tumours with chemotherapy which kills cancer cells in the bone marrow and normal cells |
Bone Marrow Transplant Patients
| 75% to 100% |
Patients receiving stem-cell transplantation so they can make new blood cells again (chemotherapy is used to prepare the patients system beforehand). |
Radiotherapy patients
| 85% to 100% |
Patients getting radiation therapy (radiotherapy) for head and neck cancer. |
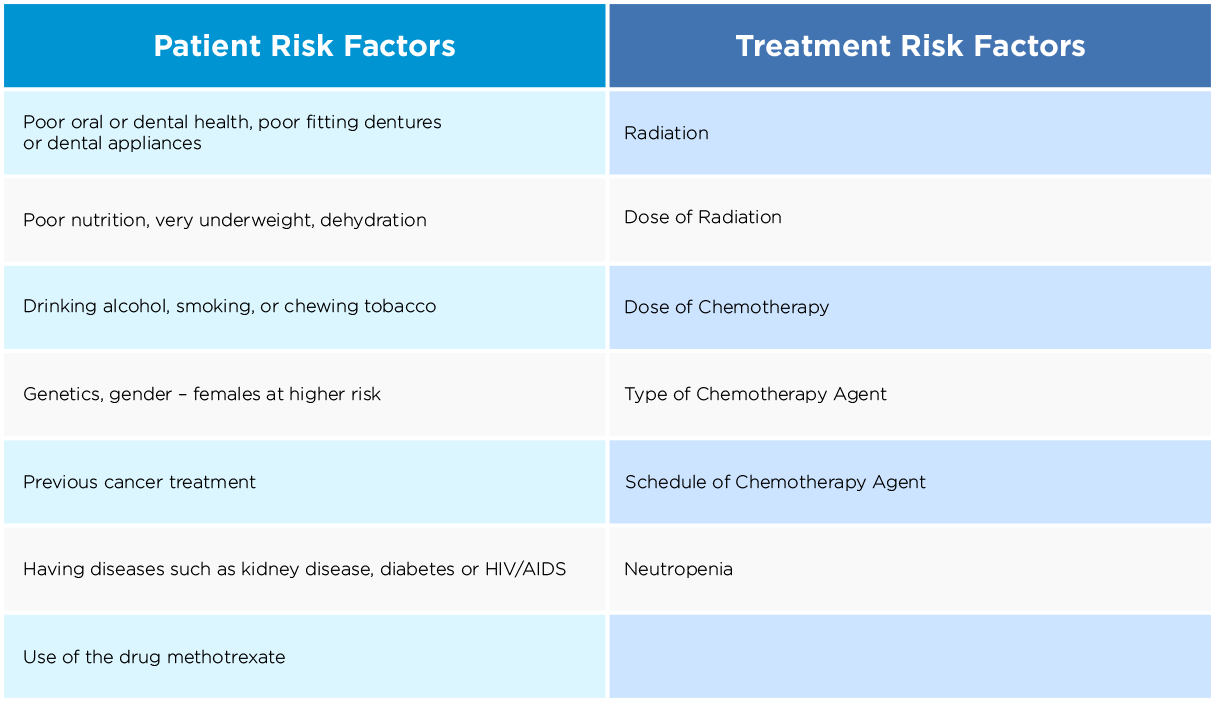
Risk Factors for Oral Mucositis
Patients’ perception of OM1,6,7,8
Oral mucositis (OM) is a significant and debilitating side effect of the oral mucosa directly caused by the mucosal toxicity of chemotherapeutic agents and radiotherapy. Mucositis causes pain that gives patients trouble eating, drinking, swallowing and speaking. It restricts the type or quality of food patients can have and can result in rapid weight loss, lead to parental feeding, the use of antibiotics and narcotics, breaks in their cancer therapy and/or hospitalization with impacts on patient survival.1,6
Oral mucositis is clearly a side effect that patients fear. From the patient’s perspective, oral mucositis lasts much longer than the impression given to patients when they first learn of the side effect.7,8
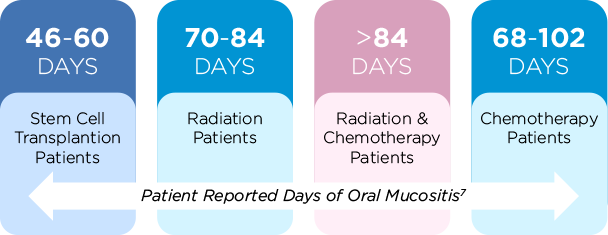
Patients’ perception of OM8
Patient VAS* ratings of side effects of chemotherapy
*VAS = Visual analogue scale
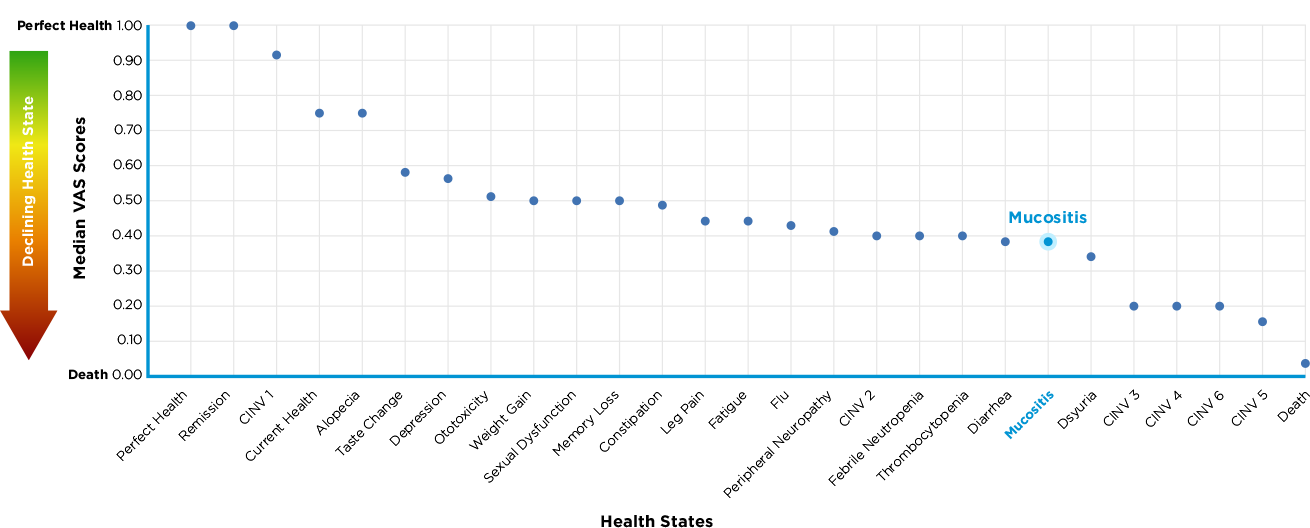
As seen in this chart, patients felt only various levels of chemotherapy induced
nausea and vomiting side effects were worse than mucositis.
This reinforces again how important it is to control patients oral mucositis.
Costs of Oral Mucositis9
Oral mucositis has clear clinical impact on patients and affects their quality of life. The economic impact of oral mucositis can also be quite impactful, especially when it is severe. A US study reviewed the supportive care costs of severe radiochemotherapy induced mucositis and pharyngitis in patients with head and neck cancer (HNC) or non small cell lung cancer (NSCLC).
The median incremental cost for patients developing severe oral mucositis/pharyngitis after cancer treatment were:
• USD $17,244 for patients with HNC
• USD $25,060 for patients with NSCLC
Most of this increase in cost was associated with inpatient hospitalization and days of stay required for supportive care (primarily alimentation) which was 2.8 times higher in patients with severe oral mucositis/pharyngitis.
However true costs should also include indirect costs:
• Time lost from work for patients
• Time lost from work by caregivers
• Additional hospital staff time to manage
• Additional supportive care pharmaceuticals as patients consume more of these items seeking symptom relief
Minimizing the impact of oral mucositis on patients has benefits to the patient, their caregivers and the healthcare system.

Gelclair® Background
- Gelclair® has been evaluated in 22 clinical studies in more than 2,000 patients
- Gelclair® has been studied in OM patients undergoing RT, CT or both, BMT, pediatric patients and patients with painful lesions of other origins
- Available in the UK for over 20 years
- On the NHS formulary10
- 1st line standard of care for OM
- Studies evaluated reduction of pain, recovery of functionality, reduction of severity of OM, impact on QOL, patient acceptability
- Recommended by UK Oral Mucositis in Cancer Group11
- Recommended by ESMO and the EOCC12,13
- Millions of doses in over 30 countries

Adapted from NHS Guidelines for the Oral Care of Patients Receiving Systemic
Anti-Cancer Treatment
What is Gelclair®?
Gelclair® is a viscous gel specially formulated to aid in the management of lesions of the oral mucosa. It forms a protective film that, by coating and sticking to the lining of the mouth, offers rapid and effective pain management.
Gelclair is a non-prescription Class II Medical Device.
Gelclair’s® protective coating14,15:
Gelclair® works by forming a protective film barrier that coats the lining of the mouth. It covers and protects the exposed nerve endings that cause pain. This stops irritation and reduces pain. Gelclair® also hydrates and helps seal moisture in the mucosal cells. Gelclair’s main ingredients are Polyvinylpyrrolidone (PVP), Hyaluronic acid and Glycyrrhetinic acid.
Polyvinylpyrrolidone (PVP)
A hydrophilic polymer with mucoadhesive and film forming properties that boosts the action of sodium hyaluronate. PVP forms a mucoadhesive barrier layer that covers oral mucosa lesions, protecting sensitive nerve endings and providing pain relief.
The barrier layer:
• Absorbs mechanical stress
• Boosts the actions of Sodium Hyaluronate.
• Has a weak antibacterial effect
Hyaluronic acid (present as Sodium Hyaluronate)
Hyaluronic acid is naturally present in most cells in the human body. It attracts water and has film forming abilities that allow it to help keep the water in the cell and provide some lubrication. This helps make the mouth feel more comfortable since the salivary glands are damaged during cancer therapy and cannot produce as much saliva.
Hyaluronic acid also has some anti-inflammatory action to reduce swelling and it facilitates healing actions inside cells.
Glycyrrhetinic acid – a metabolite of licorice, used as a flavouring agent.
Gelclair® provides a simple, effective way to rapidly relieve the pain of oral mucositis and painful mouth ulcers while helping you to eat and drink normally.
In clinical studies, Gelclair® effectively reduced mouth pain and provided rapid relief that helped cancer patients with oral mucositis to eat and drink more easily which improves your quality of life and helps you to stay on your cancer therapy.
Gelclair®: Rapid Pain Relief From Oral Mucositis for Over 20 Years
- Overall efficacy results show relief from pain and improvement in functionality in patients with oral mucositis.
- Pain relief equal to recommended comparators, with differences favoring Gelclair® in either relief from pain, pain on swallowing, and pain on speaking though not statistically significant.
- Reported decrease in need for analgesics/opiates.
- Gelclair® demonstrated significantly better patient tolerability and acceptability than standards of care.
- No reported drug interactions and no serious side effects reported.
- With over 20 years of experience, Gelclair® has demonstrated it is an effective product in protecting patients from the pain associated with oral mucositis caused by radiotherapy or chemotherapy and improved their ability to eat, drink, swallow and speak.
Clinical Studies Overview
Study 1
Gelclair® Effective Pain Reduction1
Efficacy of Gelclair® in Reducing Pain in Palliative Care Patients with Oral Lesions,
Innocenti M et al, J Pain Symptoms Management, 2002;24(5):456-7
Objective
To evaluate the effectiveness of Gelclair® in patients suffering from inflammatory oral pathologies of various etiologies.
30 in-patients, 30-60yrs age, life expectancy of a few months, various inflammatory pathologies of the oral cavity from various etiology
Results
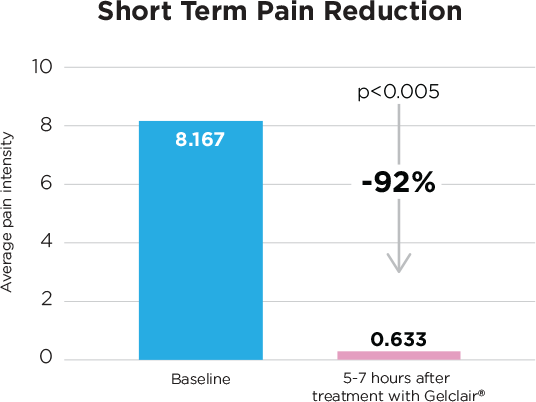
- Gelclair® significantly reduced pain by 92% from baseline (p<0.005) in the short term (5-7 hours)
- After 7 days of Gelclair®, 87% reported there was:
- Overall improvement in pain caused by swallowing saliva, liquids and food
- Significant improvement in ability to swallow saliva, drink liquids and eat semisolid foods and foods from a normal diet.
- Gelclair® was easy to use and well tolerated by all.
Conclusions
- Gelclair® controls pain and improves the ability to eat and drink in patients with painful illnesses of the oral mucosa, such as oral mucositis.
Study 2
Decrease in Pain in OM RT Patients2
The clinical effectiveness of Gelclair® in the management of oral mucositis
Lindsay G et al. Aust Nursing J. 2009;16:33.
Objective
Gelclair’s® effect on pain scores in HNC patients with OM receiving RT
Methods and Patients
- 33 patients 18-81 yrs
- Pain measured by VAS pain scale pre-Gelclair® treatment, and one hour after Gelclair® treatment.
Results
- Gelclair® used an avg of 2.29 days, max of 4 days.
- Pre-Gelclair® treatment, 88% of patients graded a pain score of 7 or above, classed as severe pain.
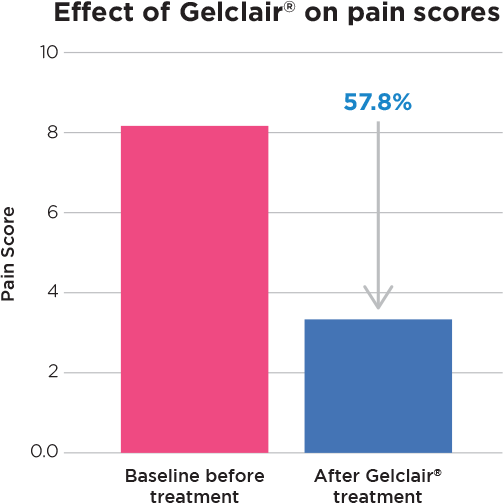
- After Gelclair® 85% of patients reported improvement in pain scores.
After treatment with Gelclair®
- 85% of patients had pain decrease
- Avg Pain score decreased by 57.8%
- 15% of patients stated they were better able to eat and drink
Study 3
Reduction in Pain and Analgesic Doses3
McLean M., An Audit of the Efficacy of Gelclair® for Mouth Pain in Patients Undergoing Radiotherapy or Chemotherapy., Presented at British Association of Head and Neck Oncology Nurses (BAHNON) National Study Day on Sharing Good Practice in Head and Neck Cancer Nursing, 12 June 2009, Leeds, UK.
Study-Design
- 26 patients, 7 day, 3x day, Grade II OM. 16 on Gelclair®
- Primary endpoint: Pain relief at 7 days.
- Secondary endpoints: Functionality – ability to eat/drink comfortably after 7 days.
- Concomitant analgesic consumption: Stable or reduced oral analgesia over 7-day period
Main Results
- 25 patients to day 3, 16 to day 7.
- Day 3 avg pain score went from 6.6 to 2.4, a decrease of 36% (p<0.0021).
- Day 7 avg pain score was down to 2.4 a decrease of 63% (p<0.0001).
- Functionality by day 3, avg score reduced from 4.2 to 3.6 for a reduction of 14% and to 17% by day 7 with lower numbers meaning improved function.
- Gelclair® significantly reduced pain of OM in Cancer patients taking RT or CT (p<0.00001)
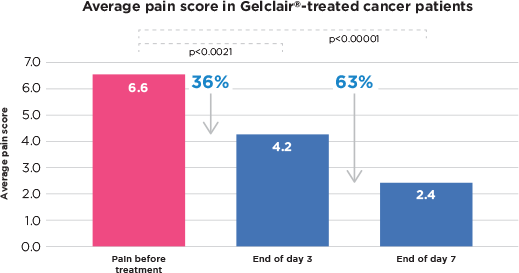
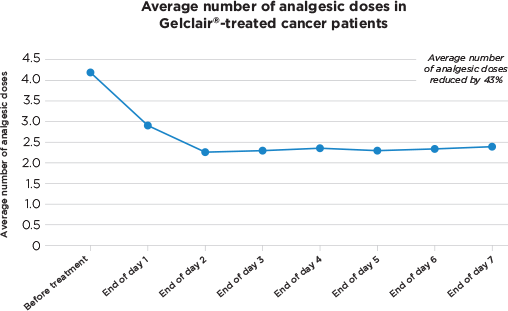
Gelclair® treatment resulted in a reduced analgesic consumption and enabled more comfortable intake of food and fluids. Gelclair® was well tolerated, with no adverse events reported.
Improvements in Pain Relief & Function
Study 4
Improvements in Pain Relief and Functionality4
Gelclair®: Potentially an efficacious treatment for chemotherapy-induced mucositis
4. De Cordi /D et al., GetclaintPotentially an éfficacious treatment for chemotherapy-induced mucositis- Abstract presented at 3rd Italian Anti Tumour League Congress of Professional Oncology Nurses, Conegliano, Italy. October 2001.
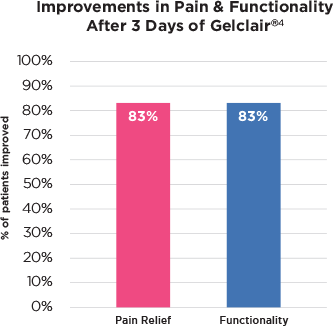
De Cordi D. et al, 2001
- Open, 30 patients, OM, CT, 3 days
- Reductions in pain relief 83% of patients
- Level of pain was reduced by 51%
- Functionality improvement in 83% of patients
Study 5
Improvements in Pain Relief and Swallowing5
Efficacy of Gelclair® oral gel in the treatment of oral mucositis in patients with head and neck tumours treated with chemotherapy and/or radiotherapy.
5.Kantardzic N, Smajlbegovic V, Kazic N, Cardzic A. Efficacy of Gelclair oral gel in the treatment of oral mucositis in patients with head and neck tumours treated with chemotherapy and /or radiotherapy. Internet Journal of Oncology 2009,6:7
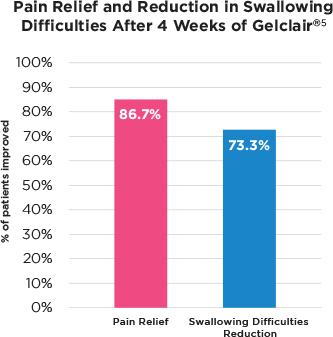
Kantardizic et al. 2009
- Open, 15 H&N patients, CT and/or RT
- Treated for OM, 1-4 weeks
- 86.7% reduction in pain across the patients
- 73.3% improvement in ease of swallowing
This product may not be right for you. Always read and follow the label and consult your healthcare provider regularly to discuss whether this medicine is working for you and if it is causing you any unwanted effects.
We cannot diagnose or prescribe treatment for individual conditions.
If you think you may have a medical condition, please visit your doctor.
References
1. Lalla R et al. Management of Oral Mucositis in Patients with Cancer , Dent Clin North Am. 2008 January ; 52(1): 61–viii, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2266835/
2. Peterson, Douglas; New strategies for management of oral mucositis in cancer patients, J Supportive Oncology, 2006 Feb 4 (2 Suppl 1):9;1, https://pubmed.ncbi.nlm.nih.gov/16499139/
3. Oral Cancer Foundation, https://oralcancerfoundation.org/complications/mucositis/
4. NHS UK Mucositis https://www.nhs.uk/conditions/mucositis/#:~:text=If%20you%20have%20mucositis%20in,your%20mouth%20moist%20(saliva%20substitutes)
5. Canadian Cancer Society – Sore Mouth and Throat, https://cancer.ca/en/treatments/side-effects/sore-mouth-and-throat
6. Lalla R et al. Management of Oral Mucositis in Patients with Cancer , Dent Clin North Am. 2008 January ; 52(1): 61–viii, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2266835/
7. McCullough, Ricky Wayne , Actual duration of patient-reported mucositis: Far longer than 2 to 4 weeks and may be avoidable altogether, Korean Journal of Clinical Oncology 2016;12:1-6 http://dx.doi.org/10.14216/kjco.16001, pISSN 1738-8082 ∙ eISSN 2288-4084
8. Charlotte C. Sun, Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer, Support Care Cancer (2005) 13:219–227, DOI 10.1007/s00520-004-0710-6
9. Nonzee et al, Evaluating the Supportive Care Costs of Severe Radiochemotherapy-Induced Mucositis and Pharyngitis Cancer 2008;113(6):1446-52, DOI 10.1002/cncr.23714
10. NHS-Guidelines for the Oral care of Patients Receiving Systemic Anti-Cancer Treatment, https://wmcanceralliance.nhs.uk/images/Documents/SaCT/Network_Guidelines_for_the_Oral_Care_of_Patients_receiving_SACT_v4.pdf
11. UK Oral Management in Cancer Care Group- http://ukomic.com/documents/oral_mucositis_products.pdf
12. D. E. Peterson et al, Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up, Annals of Oncology 26 (Supplement 5): v139–v151, 2015, doi:10.1093/annonc/mdv202, Published online 4 July 2015
13. Quin et al, EOCC – European Oral Care in Cancer Group, Oral Care Guidance and Support- Nov 2017, https://eocc.co.uk/wp-content/uploads/2018/09/EOCC-English-Guidance.pdf
14. Kowalska and U. Kalinowska-Lis, 18ß-Glycyrrhetinic acid: its core biological properties and dermatological applications, International Journal of Cosmetic Science,2019,41, 325–331doi: 10.1111/ics.12548
15. Pranav K et al, Topical Hyaluronic Acid in the Management of Oral Ulcers, Indian J Dermatol. 2011 May-Jun: 56(3):300-302 PMCID:PMC3132908 PMID:21772592, doi:10.4103/0019-5154.82485:10.4103/0019-5154.82485